Successful Synthesis of Pure And Perfectly Structured Interferon Alpha-2a by Chemical Protein Synthesis
Interferon Alpha-2a (INF Alpha-2a) is a type 1 interferon that consists of 165 amino acid residues, which is a therapeutic mid-size protein, is traditionally produced by recombinant technology. This cytokine is extensively used for its antiviral and antineoplastic properties.
1. Chemical Protein Synthesis
Chemistry offers endless possibilities for producing custom proteins. Our technology makes it possible to build molecules atom by atom and precise product adjustments can be considered. In addition, our staff with more than 20 years of experience guides you to make the best choices.
- Our synthesis
Thanks to our unique and proprietary technology, we have pushed the limits of peptide chemical synthesis higher and further. We can now produce mid-size proteins containing 70 to 400 amino acids.
- CO-development
We team-up with our partners, from the very outset by working closely together, designing, customizing and optimizing fully-active leads for their pre-clinical development and beyond.
- Bio-Betters
Thanks to the evolving regulations and our advanced technology, we can now think about biosimilar drugs free of endotoxins and without other rDNA production contaminants and impurities i.e. “clean” biosimilars or “Biobetters.”
Our chemical synthesis platform aims to deliver mid-size proteins with the highest of purity and homogeneity, at milligrams scale from early R&D stage up to Industrial full GMP production at multi-grams scales.
Based on the well-known ligation technology, we have developed proprietary processes and linkers allowing the assembly of peptidic fragments very specifically, block by block.
We can produce not only native proteins but also precisely modified proteins. A large panel of modifications are compatible with our skills and technologies:
- Phosphorylation / Glycosylation / PEGylation
- Labelling (Fluorophores, biotin, quenchers)
- Un-natural amino acids (D-amino acids, isotopic labelling N15 and C13…)
- Fusion proteins, cyclic proteins
By building those custom proteins, atom by atom, we ensure localization of any modifications with absolute precision. Proteins synthesized chemically are inherently of very high quality and purity. They have no isoforms, no endotoxins, no biological contaminants.
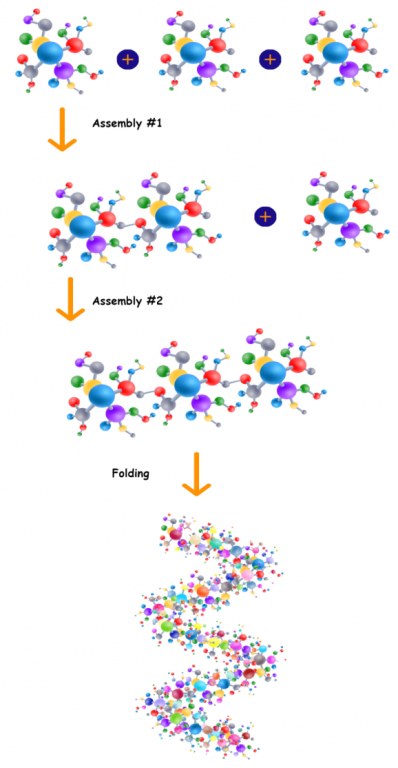
Schematic synthesis strategy:
Synthesis of linear fragments of protein
- Using solid phase peptides synthesis (SPPS) strategy
- Purification of fragments using RP-HPLC

Fragments Assembly
- Using Assembly of linear peptides using our proprietary technology
- One-pot reaction without intermediary purification
- Purification of linear protein using RP-HPLC
Cheminal protein synthesis advantages:
- Proteins with maximized biological activity
- Can bypass the problems with the biological proteins production (toxicity, purity, inclusion bodies…)
- Can helps with complex proteins that cannot be produced by biological systems (high hydrophobicity, intermenbrane loop)
- Very high purity (Endotoxin, Animal and Biological Free)
- Homogeneity in Preparations (no isoform)
- For proteins that need un-natural amino acids or finely controlled sidechains
- Automated production at milligrams and scale-up to Industrial scale
2. INF Alpha-2a chemical synthesis
- Assembly strategy
We employed a 3-fragments assembly strategy in order to ensure optimized yield and purity. Fragments were synthetized by classical Solid Phase Peptide Synthesis with our proprietary resin and purified with RP-HPLC.
The assembly of the linear protein used our proprietary technology in a one-pot reaction, without any intermediary purification. Purification of the linear protein was done by RP-HPLC.


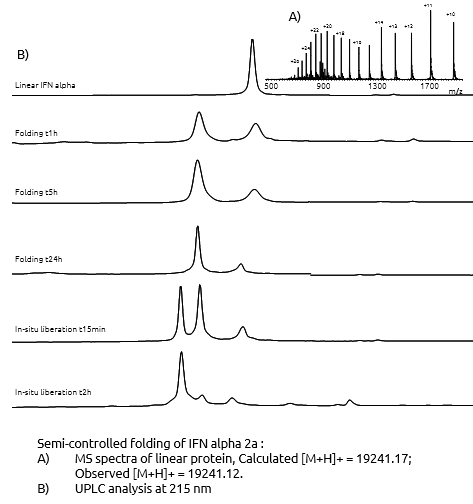
- Semi controlled folding
The assembly By using modification on the protein sequence, we perfomed a highly efficient folding in solution, leading to high yields. After completion of the folding step, the liberation of the native folded protein was done by in-situ treatment. The final purification of the protein was performed by PR-HPLC or HIC methods
- Final characterisation
To ensure optimal purity and correct structure of Interferon Alpha-2A had been achivieved, we analysed this mid-size protein with the highly reliable chromatohraphic and mass spectrometry methods, which we have developed.
Strong QA/QC endured a purity in excess of 95 % of the final Interferon Alpha-2A
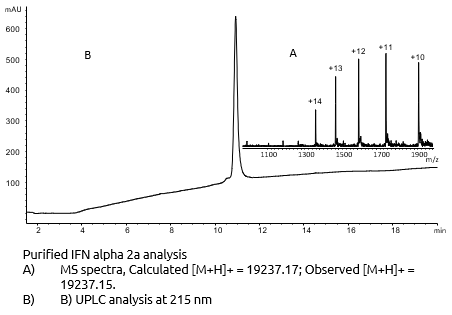
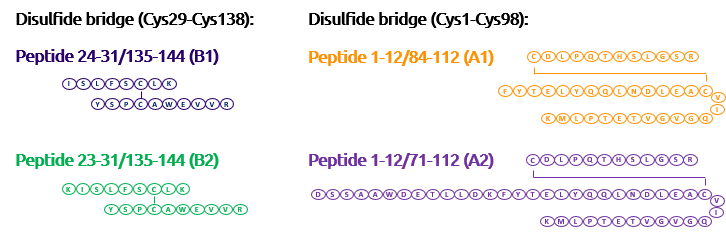
- Structural Characterisation
Interferon Alpha-2A is know to have 2 disulfide bonds: Cys1-Cys98 and Cys29-Cys138. We demonstrated the correct cysteines by enzymatic digestion (Trypsin) and UHPLC/Mass Spectrometry analysis of the resulting fragments.
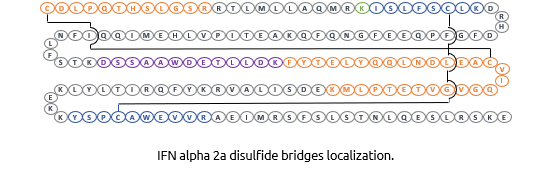
- Analysis of IFN Aplpha-2A by LC-MS
IFN Aplpha-2A was enzymatically cleaved
The fragments were determined and correct disulfide bonds were confirmed
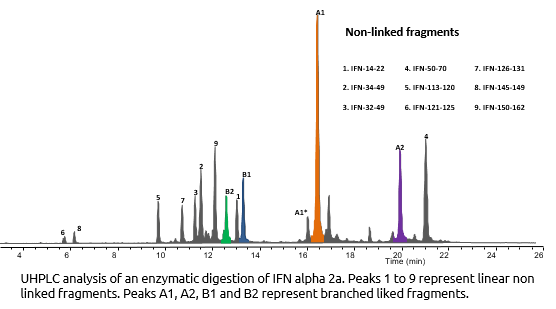
- Linked peptide fragment identifictaion